Abstract
Background: SNDX-5613 is a potent, orally available, small molecule inhibitor of menin, which binds to MLL1, encoded by the KMT2A gene, and MLL1 fusion proteins. Menin expression leads to aberrant gene transcription through its activation of Homeobox-related genes, leading to maturation arrest and leukemogenesis in KMT2A-rearranged and NPM1-mutated leukemias. Inhibition of menin activity significantly blocks the transcriptional signature and subsequently the proliferative capacity of leukemia cells harboring NPM1 mutations or KMT2A rearrangements. SNDX-5613 and its analogs have demonstrated robust single-agent anti-proliferative activity in NPM1-mutated AML cells and AML patient-derived xenograft models. Currently, SNDX-5613 is being explored as a single agent in a phase 1/2 study in patients with NPM1-mutated or KMT2A-rearranged relapsed/refractory AML, mixed phenotypic acute leukemia and acute lymphoblastic leukemia. The primary objective of this study is to determine the safety and recommended dose of SNDX-5613 combined with AZA and VEN in untreated AML patients age ≥ 60 years who are not candidates for intensive chemotherapy.
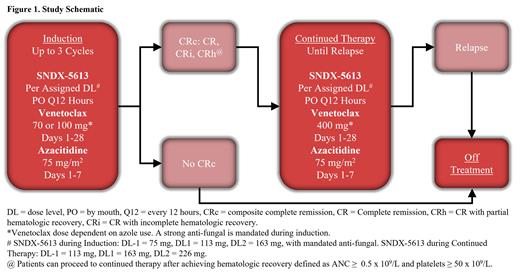
Study Design and Methods: This study is a sub-study of the BeatAML Master Trial (NCT03013998) in which untreated AML patients age ≥ 60 are assigned an investigational therapy based on cytogenetic and central genomic analysis. Patients must specifically harbor an identifiable NPM1 mutation without a concomitant FLT3 mutation or have a KMT2A rearrangement to be eligible. In this phase 1b study, the primary objective is to determine the safety and recommended dose using a standard 3+3 design based on dose-limiting toxicities with 3 dose levels (DL) of SNDX-5613 to be evaluated in combination with VEN/AZA and an anti-fungal (required during induction): 75mg (DL-1), 113mg (DL1), and 163mg (DL2). In continuation phase with anti-fungals not required, SNDX-5613 will be dosed at 113mg (DL-1), 163mg (DL1), or 226mg (DL2) with appropriate dose reductions added for both SNDX-5613 and VEN if continuing on anti-fungals. The secondary objectives are to assess the overall response rate, measurable residual disease (MRD) status, overall survival, transplant rate and duration of remission.
During induction cycles, VEN (D1-28) and AZA (D1-7) are dosed per standard of care every cycle. SNDX-5613 is dosed continuously orally twice daily with dose levels listed above. In patients who achieve a morphologic remission (<5% blasts), SNDX-5613 will be held if absolute neutrophil count (ANC) is < 0.5x109/L and/or platelets (PLT) < 50x109/L. Patients can receive up to 3 induction cycles to achieve a composite complete remission (CRc: CR, CRi, CRh). Patients can receive continuation therapy with AZA, VEN and SNDX-5613 once they achieve CRc and after hematologic recovery. Following the determination of a safe and recommended dose in phase 1b, there will be a dose expansion cohort of 12-20 patients following the same schedule as described above (Figure 1) to further evaluate safety, tolerability and correlative biomarkers.
Correlative objectives include assessment of SNDX-5613 pharmacokinetics and its correlation with response and toxicities. Additionally, the study will evaluate the impact of MRD, as measured by central flow cytometry and next-generation sequencing of NPM1 mutations. Additional evaluations and biomarkers of potential interest include menin- KMT2A-specific transcriptomic changes, myeloid differentiation/cell subset changes, mechanisms of resistance, and discovery of baseline features (methylation, mutations, etc.) that can impact outcomes.
Disclosures
Zeidner:AbbVie: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Honoraria; Genentech: Honoraria; Immunogen: Honoraria; Servier: Consultancy, Honoraria; Shattuck Labs: Honoraria; Arog: Research Funding; Astex: Research Funding; Jazz: Research Funding; Merck: Research Funding; Stemline: Research Funding; Sumitomo Dainippon Pharma: Research Funding; Syndax: Research Funding; Takeda: Research Funding. Foster:LOXO Oncology: Research Funding; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bellicum Pharmaceuticals: Research Funding; Macrogenics: Research Funding; Newave Pharmaceuticals: Research Funding; Rafael Pharmaceuticals: Research Funding; Zentalis Pharmaceuticals: Consultancy. Huang:AstraZeneca: Other: Statistical support. Stein:PTC Therapeutics and Syros: Membership on an entity's Board of Directors or advisory committees; Bayer: Research Funding; Syndax: Consultancy, Research Funding; Amgen, AbbVie, Seattle Genetics, and Biotheryx: Consultancy; Auron Therapeutics: Current equity holder in private company; PinotBio, Bristol Myers Squibb, Jazz Pharmaceuticals, Foghorn Therapeutics, Blueprint Medicines, Gilead Sciences, Janssen Pharmaceuticals: Consultancy; Daiichi-Sankyo, Celgene Pharmaceuticals, and Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas Pharmaceutical, Agios Pharmaceuticals, and Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees. Foran:AbbVie, Actinium, Aptose, Astex, H3Biosciences, Kura Oncology, Trillium, Xencor: Research Funding; Novartis, Servier, Pfizer, BMS, Taiho: Other: Formal Advisory Activities. Baer:Kura Oncology: Research Funding; AbbVie: Research Funding; Ascentage: Research Funding; Forma: Research Funding; Takeda: Research Funding; Kite, a Gilead Company: Research Funding. Stock:Kite: Honoraria; Kura Oncology: Honoraria; MorphoSys: Honoraria; Jazz Pharmaceuticals: Honoraria; Amgen: Honoraria; Agios: Honoraria; Servier: Honoraria; Pluristem: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Syndax: Consultancy, Honoraria; Newave Pharmaceuticals: Consultancy. Madanat:Sierra Oncology, Stemline Therapeutics and Novartis: Membership on an entity's Board of Directors or advisory committees; BluePrint Medicines, GERON, OncLiv: Consultancy, Honoraria. Kovacsovics:Abbvie: Research Funding; Caelum: Research Funding; Gilead: Research Funding; Glycomimetics: Research Funding; Janssen: Research Funding; Jazz Pharmaceuticals: Research Funding; Novartis: Honoraria, Research Funding; Syndax: Research Funding; Kite: Honoraria. Olin:Cellectis: Other: NA; Actinium: Consultancy; Astellas: Consultancy; Abbvie: Consultancy. Schiller:Celgene: Consultancy, Research Funding, Speakers Bureau; Cyclacel: Research Funding; CTI: Research Funding; Stemline: Research Funding; Ono Pharma: Honoraria; AstraZeneca: Honoraria; Medimmune: Research Funding; Deltafly: Research Funding; Johnson & Johnson: Current equity holder in publicly-traded company; Glycomimetics: Research Funding; Sellas: Research Funding; Trovagen: Research Funding; AltruBio: Research Funding; Deciphera: Research Funding; PreCOG LLC: Research Funding; FujiFilm: Research Funding; Samus: Research Funding; Millennium: Research Funding; Cellectis: Research Funding; Constellation: Research Funding; Onconova: Research Funding; Novartis: Honoraria, Other: Speaker fees, Research Funding; AVM Biopharma: Research Funding; Daiichi-Sankyo: Research Funding; Genentech-Roche: Research Funding; Geron: Research Funding; Cellerant: Research Funding; Bristol Myers Squibb: Current equity holder in publicly-traded company, Speakers Bureau; Pfizer: Research Funding; Jazz: Consultancy; Kite, a Gilead Company: Research Funding, Speakers Bureau; Stemline: Speakers Bureau; Actuate: Research Funding; Arog: Research Funding; Gilead: Research Funding; Amgen: Current equity holder in publicly-traded company, Honoraria; Mateon: Research Funding; Regimmune: Research Funding; Janssen: Research Funding; Forma: Research Funding; Karyopharm: Research Funding, Speakers Bureau; Astellas: Research Funding, Speakers Bureau; Actinium: Research Funding; Gamida: Research Funding; Incyte: Other: speaker fees, Research Funding, Speakers Bureau; Agios: Consultancy, Honoraria; AbbVie: Research Funding, Speakers Bureau; Sangamo: Research Funding; Takeda: Research Funding; Tolero: Research Funding. Lin:AbbVie, Aptevo, Astellas Pharma, Bio-Path Holdings, Celgene, Celyad, Genentech-Roche, Gilead Sciences, Incyte, Jazz Pharmaceuticals, Mateon Therapeutics, Ono Pharmaceutical, Pfizer, Prescient Therapeutics, Seattle Genetics, Tolero, Trovagene: Research Funding. Redner:Pfizer: Current equity holder in publicly-traded company. Curran:Amgen: Consultancy, Other: Participation in advisory board; Incyte: Consultancy, Other: Participation in advisory board; Kite: Consultancy, Other: Participation in advisory board; Pfizer: Consultancy, Other: Participation in advisory board; Tempus: Consultancy, Other: Participation in advisory board; Servier: Consultancy, Honoraria, Other: Participation in advisory board; participation in expert panel guidelines. Levine:Qiagen: Other: supervisory board member; Imago, Mission Bio, Bakx, Zentalis, Ajax, Auron, Prelude, C4 Therapeutics and Isoplexis: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Ajax, Abbvie, Constellation, Zenalis, Celgene, Roche, and Prelude: Other: research support; Gilead and Novartis: Other: Grant reviews; Astra Zeneca and Kura: Other: honoraria for invited lectures ; Syndax, Incyte, Janssen, Astellas, Morphosys and Novartis: Consultancy. Druker:Adela Bio: Other: Scientific Advisory Board ; Cepheid: Other: Scientific Advisory Board ; Therapy Architects (ALLCRON): Other: Scientific Advisory Board ; Aileron Therapeutics: Other: Scientific Advisory Board ; CytoImage: Patents & Royalties: QD Molecular Assay for Personalized Oncoprotein Detection in Leukemia (exclusive license); Sun Pharma Advanced Research Company: Patents & Royalties: Mutated ABL Kinase Domains (non-exclusive license); (Novartis exclusive license) and OHSU and Dana-Farber Cancer Institute (one Merck exclusive license, one CytoImage, Inc. exclusive license, and one Sun Pharma Advanced Research Company non-exclusive license): Patents & Royalties: Patent 6958335; Merck: Patents & Royalties: Monoclonal antiphosphotyrosine antibody 4G10; Celgene: Other: Scientific Advisory Board ; DNA SEQ: Other: Scientific Advisory Board ; Nemucore Medical Innovations: Other: Scientific Advisory Board ; Novartis: Other: Scientific Advisory Board; Clinical Trial Funding , Patents & Royalties: 6958335 (exclusive license), Research Funding; RUNX1 Research Program: Other: Scientific Advisory Board ; Aptose Biosciences: Current holder of stock options in a privately-held company, Other: Scientific Advisory Board ; Blueprint Medicines: Current holder of stock options in a privately-held company, Other: Scientific Advisory Board ; Enliven Therapeutics: Current holder of stock options in a privately-held company, Other: Scientific Advisory Board ; Iterion Therapeutics: Current holder of stock options in a privately-held company, Other: Scientific Advisory Board ; GRAIL: Current holder of stock options in a privately-held company, Other: Scientific Advisory Board ; Recludix Pharma: Current holder of stock options in a privately-held company, Other: Scientific Advisory Board ; Amgen: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Vincerx Pharma: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Burroughs Wellcome Fund: Membership on an entity's Board of Directors or advisory committees; CureOne: Membership on an entity's Board of Directors or advisory committees; Beat AML LLS: Other: Joint Steering Committee ; Multicancer Early Detection Consortium: Membership on an entity's Board of Directors or advisory committees; VB Therapeutics: Other: Founder; Enliven Therapeutics: Other: Sponsored Research Agreement ; Recludix Pharma: Other: Sponsored Research Agreement ; Astra-Zeneca: Other: Clinical Trial Funding, Research Funding; US Patent: Patents & Royalties: 4326534; US Patent: Patents & Royalties: 6958335; US Patent: Patents & Royalties: 7416873; US Patent: Patents & Royalties: 7592142; US Patent: Patents & Royalties: 10473667; US Patent: Patents & Royalties: 10664967; US Patent: Patents & Royalties: 11049247. Borate:Genentech: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees; AbbVie/Genentech: Membership on an entity's Board of Directors or advisory committees. Byrd:Pharmacyclics LLC: Honoraria, Research Funding; Syndax: Consultancy; Xencor, Inc: Research Funding; AstraZeneca: Consultancy; Janssen Pharmaceuticals, Inc.: Consultancy; Kura Oncology, Inc: Consultancy; TG Therapeutics: Honoraria; Vincerx Pharma: Current equity holder in publicly-traded company; Novartis: Consultancy, Honoraria. Mims:Daiichi Sankyo: Other: Data Safety and Monitoring Board; Jazz Pharmaceuticals: Other: Data Safety and Monitoring Board; BMS: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees; Ryvu: Membership on an entity's Board of Directors or advisory committees; Zentalis: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.